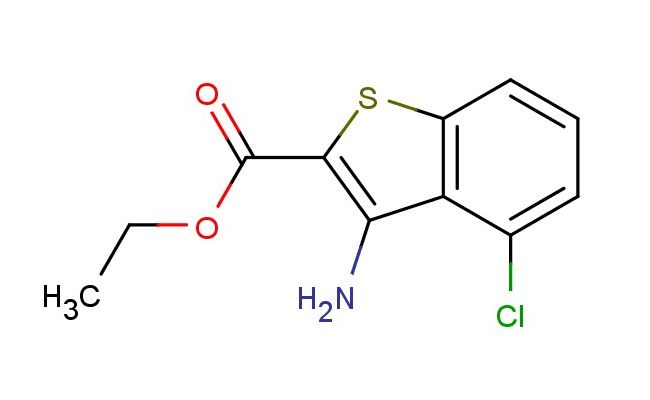
ethyl 3-amino-4-chlorobenzo[b]thiophene-2-carboxylate
$300.00
CAS No.: 67189-92-8
Catalog No.: 194497
Purity: 95%
MF: C11H10ClNO2S
MW: 255.726
Storage: 2-8 degree Celsius
SMILES: C(C)OC(=O)C1=C(C2=C(S1)C=CC=C2Cl)N
Catalog No.: 194497
Purity: 95%
MF: C11H10ClNO2S
MW: 255.726
Storage: 2-8 degree Celsius
SMILES: C(C)OC(=O)C1=C(C2=C(S1)C=CC=C2Cl)N
CAS NO.: 67189-92-8; ethyl 3-amino-4-chlorobenzo[b]thiophene-2-carboxylate. PROPERTIES: ethyl 3-amino-4-chlorobenzo[b]thiophene-2-carboxylate is a crystalline solid. Its molecular formula is C11H10ClNO2S, and the molecular weight is approximately 263.73 g/mol. The compound has a melting point of approximately 80-82°C. It is moderately soluble in common organic solvents such as ethyl acetate and tetrahydrofuran, but is poorly soluble in water. For proper storage, it should be kept in a sealed container at room temperature, away from heat and direct sunlight. As a compound containing a benzo[b]thiophene ring, an amino group, a chlorine atom, and an ester group, it may exhibit certain reactivity and stability. When handling it, protective gloves and eye protection should be worn. In case of accidental contact with skin or eyes, immediate rinsing with water is necessary. APPLICATIONS: In organic synthesis, ethyl 3-amino-4-chlorobenzo[b]thiophene-2-carboxylate serves as a valuable intermediate. The amino group can undergo various reactions such as acylation, sulfonation, and diazotization. The chlorine atom provides a site for substitution reactions. The ester group can be hydrolyzed to a carboxylic acid. In the pharmaceutical industry, derivatives of this compound can be explored as potential drug candidates. For example, in the development of certain anti-inflammatory drugs, the structure of ethyl 3-amino-4-chlorobenzo[b]thiophene-2-carboxylate can be modified to enhance the drug's efficacy and reduce side effects (as reported in medicinal chemistry journals). Additionally, in the field of materials science, it can be incorporated into the synthesis of functional materials such as liquid crystals or organic semiconductors, where its structure can influence the material's electronic and optical properties (as noted in materials chemistry research papers).
Reviews
Write Your Own Review




![3,6-dichlorobenzo[b]thiophene-2-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/194479_1.jpg)
![5-(tert-butyl)benzo[b]thiophene](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195029_1.jpg)